Starting your research career can be daunting, as you assemble the team and resources you need to succeed. Access to sophisticated equipment, specialized personnel and standardized procedures is needed for successful grant funding and publication. CADRE’s multidisciplinary Clinical Lab Core (CLC) makes these resources available to early career investigators in areas like substance use and metabolic health, lowering the barrier to entry for complex human subjects research.
One-Stop Shop for State-of-the-Art Data Collection
Led by Dr. Jennifer (Jen) Tidey and staff members Ms. Carline Fleig, NP, APRN, and Ms. Christine Goodwin, M.Sc., LMHC at the Brown University School of Public Health, the CLC offers clinical research support, advanced in-house capabilities and equipment designed to enhance the quality and efficiency of clinical research studies.
Expert Clinical and Assessment Services
The CLC can provide essential support for human subjects research, including:
- Physicals and clinical monitoring
- IV starts and blood draws
- Mental health safety plan
The CLC offers a safety plan and staff trained in assessing risk for self-harm or suicidal ideation—an important service for studies involving vulnerable populations. As Christine states, “What we are very proud of is our mental health safety plan… and assessing risk for self-harm or suicidal ideation.”
Advanced Equipment and Biomarker Processing
- ELLA Instrument: This platform allows the core to process over 200 biomarkers in-house, significantly reducing the time and cost associated with sending samples to external labs. The design of this instrument “provides highly reproducible and accurate data for our PIs.”
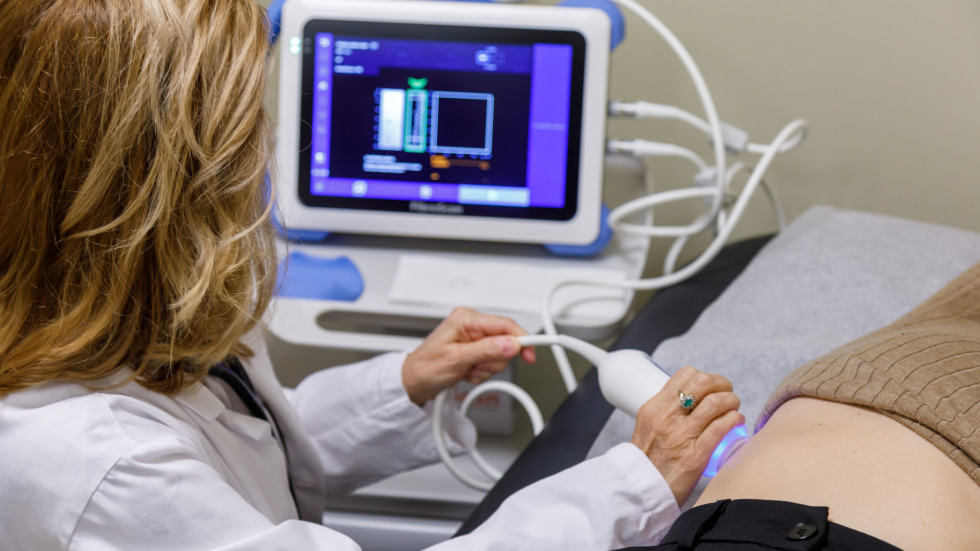
- FibroScan Instrument: The portable FibroScan is administered by trained staff and can be used on site or off site. It measures liver health, providing two key metrics:
- CAP (Continuous Attenuation Parameter) Score: Measures the amount of fat in the liver
- E Score: Measures liver stiffness
The FibroScan is particularly in demand given high current interest in Metabolic Dysfunction-Associated Fatty Liver Disease, which is strongly linked to obesity, type 2 diabetes, and alcohol use disorder. CLC Director Tidey highlighted this, stating it is "a hot topic" because of comorbid risk factors like alcohol use and obesity.”
Three Ways the CLC Lowers Barriers
The CLC is committed to making complex research feasible and cost-effective for all investigators.
- Early Consultation and Feasibility Assessment
Reach out during study design: Investigators are strongly encouraged to contact the CLC as they are designing their study. Jen urged, “Reach out to us. We can help you think through the types of data you want to collect, the timepoints, and the relevant biomarkers.”
Feasibility triage: The team can provide input on the feasibility of your proposed study design. Carline cannot stress enough the value of meeting early, adding, “I think we can have some really important input when it comes to, wow, is this going to actually be feasible? And if it isn’t in the way they’ve thought about it initially, maybe we can tweak it so that it can be more feasible.”
- Cost-Effective, Flexible Services
The CLC uses a fee-for-service model where services are priced on a per-participant, per-hour, or per-session basis, eliminating the need to budget for a large percentage of a dedicated staff member’s salary. The CLC is not a for-profit business, ensuring that rates are as low as possible. This is particularly helpful for early-career investigators, who often have smaller grant budgets. In addition, Carline shares that “Because we can buy our supplies in greater bulk and everything is one-stop shopping, that’s where it becomes cost effective to go through our [core].”
- Standardized Protocols and Navigation Support
The CLC has developed over 40 researcher-facing Standard Operating Procedures (SOPs) to ensure that assessments are reliable and valid. The core’s questionnaire bank includes resources such as questionnaires translated into Spanish to support studies seeking to include Spanish-speaking populations. In addition, the core can help researchers access other university resources.
Adapting to the Future: The Rise of Remote Studies
Stemming from the necessity of social distancing during the COVID-19 pandemic, the CLC has committed to supporting clinical research conducted remotely, a trend in human subjects research. In this way, more participants can be enrolled in studies to achieve greater variety in the participant pool. Technologies for remote physiological and biological specimen collection are rapidly evolving, and tools like Zoom can be used to observe and instruct participants remotely on data collection.
Collaboration in Action
Aid is not a one-way street at the Clinical Lab Core. Dr. Mollie Monnig, one of the original investigators on the COBRE grant, has helped to shape how the CLC works.
Dr. Monnig’s work involves the gut microbiome, HIV, inflammation, and alcohol use. Carline also noted that Dr. Monnig “has run the gamut of all of our services,” including the FibroScan, the ELLA instrument, blood draws, and IV starts. Dr. Tidey added, “She’s a great success story because it was a win both for us and for her. She was one of the original investigators on the COBRE grant under which the core was developed. She really pushed us… It helped us to envision what we could do.”
Takeaway for Early Career Investigators
The biggest takeaway is: Reach out early!
Don’t assume that the CLC can’t accommodate a service. “Even if it’s not specifically a thing that we’re advertising, if it’s something that’s feasible… we can do it.” Engaging with the core team early in your study design is the most effective way to ensure a feasible, cost-effective, and methodologically rigorous research project. Learn More about the CLC.